THERACURMIN®
Turmeric & Curcumin
What is turmeric?
Turmeric is a perennial plant of the ginger family and is widely spread throughout South and Southeast Asian countries. It is commonly used as a spice, flavoring agent, food preservative, and coloring agent, and is known as a key ingredient of curry powder.

What is curcumin?
Curcumin is a polyphenol contained in the rhizome of turmeric and is considered to be a main active ingredient of turmeric. It has a bright yellow color and is used as a natural food dye.
It is also well known for its effect of anti-inflammatory and anti-oxidant amongst medical and health science laboratories all over the world. A great number of researches are being conducted by a variety of medical and science institutes.
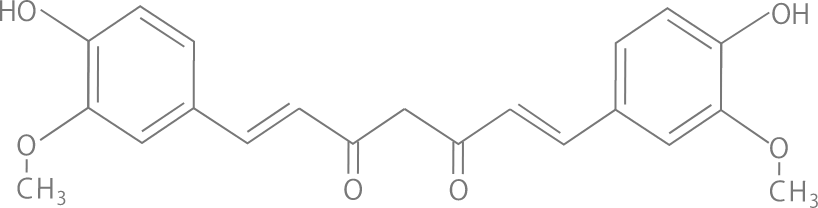

What is THERACURMIN®?
There is a problem of low absorption and bioavailability of the curcumin used in foods with function claims and supplements.
THERACURMIN® is a curcumin formulation that improves the problem of low absorption and bioavailability.
Submicron particle
of curcumin
Surface preparation for
preventing agglomeration
THERACURMIN®
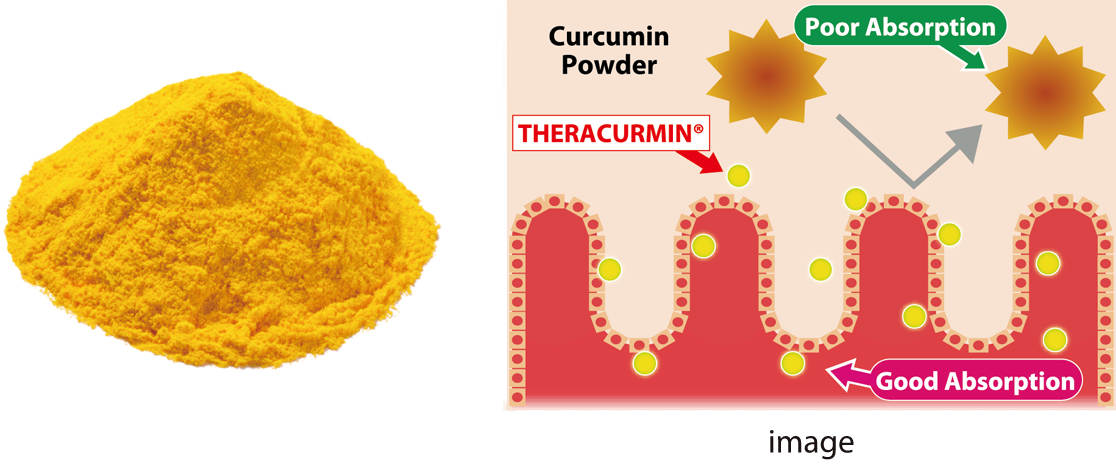
Curcumin Powder

THERACURMIN®
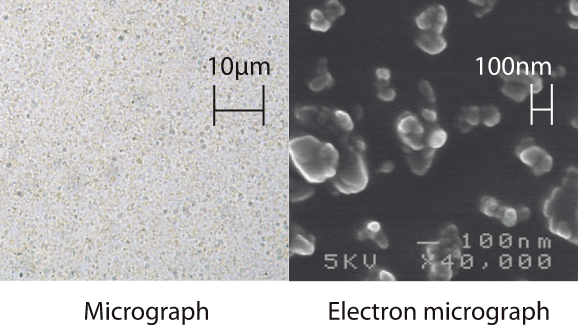
Comparison in
curcumin dispersion

Water dispersion comparison
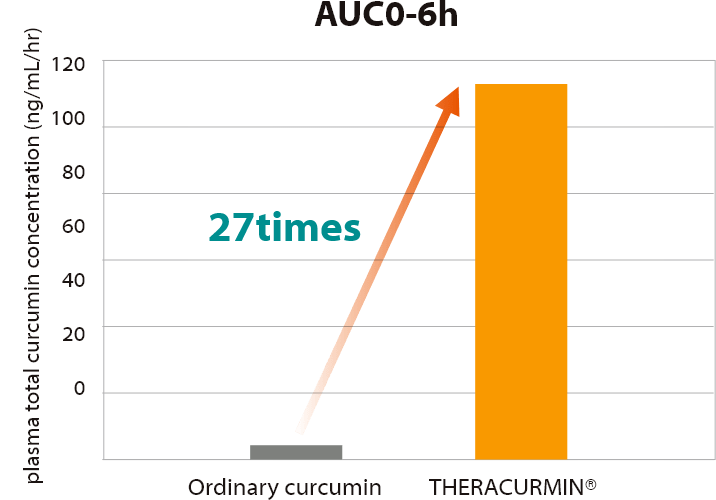
THERACURMIN® possesses high bioavailability.
Area Under the Curve(AUC)0-6h values of THERACURMIN® were 27-fold higher than those of curcumin powder.
AUC0-24 h was found to be 11.0- and 5- fold higher with TEHRACURMIN® than Company A and Company B, respectively.
Study Protocol
Study design:
Two arms, randomized parallel-group controlled trial
Subjects:
14 healthy subjects (male/female=8/6, age: 44.1±8.5 years old)
Intake:
-THERACURMIN®:30mg -Ordinary curcumin: 30mg
Area Under the Curve(AUC)0-6h values of THERACURMIN®
were 27-fold higher than those of curcumin powder.
Study Protocol
Study design:
Three arms, randomized double-blind 3-way crossover trial
Subjects:
9 healthy subjects (male/female=5/4, age: 24-32)
Intake:
-THERACURMIN®:180mg
-Company A: 260mg
-Company B: 150mg
AUC0-24 h was found to be 11.0- and 5- fold higher
with TEHRACURMIN® than Company A and Company B, respectively.

Line up
THERACURMIN® CR-033P
A highly absorbent curcumin preparation used as a raw material of functional food.
By crumbling the particles of curcumin and maintaining their particle sizes stably, the particles are absorbed by approximately 27-fold thanks to our original patent technology (Patent No. 5448511). As it also has water dispersion capability and heat and light resistance characteristics, it is applicable to any forms of products as shown below.

Application
- Hard capsule
- Tablet
- Granules
- Beverage, Liquid
- Gummy, chewable, gum
- Jelly
- Ice lolly, popsicle, sherbet
- Noodles
Safety and toxicity data of THERACURMIN®
Curcumin is a highly safe material which is approved by many safety reports.
The toxicity evaluation and safety test results of the developed THERACURMIN® are as follows.
| Toxicity data | ||||||
|---|---|---|---|---|---|---|
| Test | Ames test | Chromosome aberration test | Micronucleus assay test | Acute toxicity study (Rats) |
Subacute toxicity study (Rats) |
Subchronic toxicity study (Rats) |
| Test detail |
Salmonella spp. (4 strains) E.Coli |
In vitro shromosome aberration test in Chinese hamster lung cells |
Male sprague dewley rats bone merrow micronucles assay |
Dose: 1250,2500,5000mg/kg (As curcumin 375,750,1500mg/kg) Once |
Dose: 0,625,1250,2500,5000mg/kg (As curcumin 0,187.5,375,750,1500mg/kg) Period: 2 weeks |
Dose: 0,1250,2500,5000mg/kg (As curcumin 0,375,750,1500mg/kg) Period: 13 weeks |
| Result | Negative | Negative | Negative | No toxicological findings observed by oral administration | No toxicological findings observed by oral administration | No toxicological findings observed by oral administration |
| Clinical study in healthy volunteers | ||
|---|---|---|
| Test | 4-week clinical study in healthy volunteers at excessive dosage | 12-week clinical study in healthy volunteers at standard dosage |
| Test detail |
Tested dosage: 900mg/q.d./p.o. (as curcumin, 5x of standard dosage) Period: 4weeks |
Tested dosage: 180mg/q.d./p.o. (as curcumin standard dosage) Period; 12weeks. |
| Result | THERACURMIN® was proved safe in this study. | THERACURMIN® was proved safe in this study. |
- q.d.: Once a day
- p.o.: Oral intake
- ADI: Acceptable Daily Intake
- Standard dosage: 180mg/day (as curcumin)= ADI: 3mg/kg x 60kg (average adult body weight)
| Previous clinical study | ||
|---|---|---|
| Study title | Memory and Brain Amyloid and Tau Effects of a Bioavailable Form of Curcumin in Non-Demented Adults: A Double-Blind, Placebo-Controlled 18-Month Trial. | 12-week clinical study in healthy volunteers at standard dosage |
| Test detail |
Subject: Non-Demented Adults THERACURMIN® dose: 180mg/day Period: 18 months |
Subject: Patients with stage Ⅰ-ⅡCOPD THERACURMIN® dose: 180mg/day Period: 6 months |
| Result | No serious adverse effects were obsevered in this study | No serious adverse effects were obsevered in this study |
SIDI packet
The below materials are available as parts of the SIDI packet
- Manufacturing information: Name and address of manufacturing site, GMP compliance, mode of manufacturing, manufacturing process
- Physical/chemical information: Full list of components, source, country of origin, source, CAS number, percent
- Labeling information: Required finished product label statements and recommended restrictions of use
- Regulatory and compliance information: Patent coverage, nutritional information, compendial grade, regulatory status and supporting documents, BSE/TSE information, vegan or vegetarian status, allergens/hypersensitivities, chemicals used during manufacturing process, gluten status, soy status,diary status, wheat status, GMO status, tariff code for import/export
- Other product information: BATCH/Lot numbering system, BATCH definition, shelf life, recommended storage conditions, recommended transport conditions, package size
- Other contact information: Sales contact name, title, and email
EMIQ®
Line up
EMIQ® R-20 (Enzymatically Modified Isoquercitrin)
The world’s first commercialized WATER SOLUBLE flavonol glycoside with higher bioavailability.
EMIQ® is chemically alpha-glycosyl Isoquercitrin, and consists of quercetin monogulucoside with 0 – 10 of additional liner glucose moieties. EMIQ® also shows significantly greater bioavailability than other available forms. In humans, it showed a 17-fold increase in plasma concentration of quercetin compared to ordinal quercetin.
EMIQ® has GRAS self-affirmation. (N. GR000220)
